![]()

Patient Registry
What is the CreatineInfo Patient Registry and Natural History Study?
The CreatineInfo Registry & Natural History Study is a patient- and caregiver-reported registry and natural history study created by the Association for Creatine Deficiencies (ACD) and hosted by the National Organization for Rare Disorders (NORD) to advance research and empower the Cerebral Creatine Deficiency Syndromes (CCDS) community.
A patient registry is a program for the collection and dissemination of standardized information from a group of patients who share a condition or experience. A natural history study is a longitudinal study that aims to fill research gaps by helping medical researchers better understand how diseases progress over time.
Why is the CreatineInfo Registry Important?
- Improves understanding of the natural history and impact of CCDS in patients’ lives
- Provides valuable information to doctors, scientists, and industry to help them develop treatments and improve patient outcomes for the small CCDS community
- Assists ACD with representing the CCDS community accurately
- Shares community-reported recommendations and standards of care
Frequently Asked Questions
-
Why do we need a CCDS registry?
CCDS are rare disorders. Many researchers have very few to no CCDS patients in their clinics and need an accurate and robust data set to draw upon. The registry allows meaningful correlations to be found across many patients in an otherwise difficult to study disease group. Sharing your data with the registry means researchers can be successful. The registry allows CCDS patients and families to share valuable information in an international, confidential, and safe database. It is also the primary registry for creatine deficiencies.
-
Who can participate?
This registry is for all CCDS patients worldwide. Patients or caregivers of individuals with the following CCDS diagnoses can participate in this registry:
- Creatine Transporter Deficiency (CTD)
- Guanidinoacetate Methyltransferase Deficiency (GAMT)
- Arginine: Glycine Amidinotransferase Deficiency (AGAT)
-
How do I enroll in this registry?
1. Visit creatineinfo.iamrare.org and click “Register” under “Join the Registry.” You may need to first click “Accept Cookies.”
2. Create an account. With one account, you can add multiple participants (CCDS patients), as needed. Each participant will have their own surveys to take.
3. Provide consent for your participation and begin completing the registry surveys. -
Who is the survey participant?
The CCDS patient is considered the “participant.” Caregivers completing surveys on behalf of the participant are called “respondents.” Caregivers will first need to create a registry account using their own information as the caregiver. Then, caregivers will add each CCDS patient as a participant to their account.
-
Are there ever new surveys available in the registry?
Yes, ACD launches approximately 2-3 new surveys in the registry each year. When a new survey becomes available, log into your registry account at creatineinfo.iamrare.org and complete any available surveys. We encourage you to complete surveys as soon as they become available so that your data is included in any reports. Some surveys are longitudinal, which means you will be asked the same questions every 6-12 months. You will receive an email when it is time to complete one of these surveys.
-
What kinds of information will I be asked to share?
Enrolled patients and caregivers will see a number of short surveys available for them to complete. Each survey typically takes approximately 15-20 minutes to complete. Data collected in these surveys includes diagnosis and treatment, disease management, quality of life, and longitudinal information about CCDS. There is also a place to upload genetic reports and other medical reports.
-
I am the caregiver to more than one CCDS patient. Do they each need a registry account?
Caregivers can create one registry account and add multiple participants (CCDS patients) to their account. Each participant will need their own set of surveys completed.
-
Can more than one caregiver complete surveys on behalf of a CCDS patient?
At this time, only one caregiver should create a registry account and complete surveys on behalf of the CCDS patient(s).
-
How were the registry surveys developed?
Many registry surveys are developed by ACD in collaboration with research partners, clinical professionals, and CCDS families. Each of these collaborators ensure the surveys are using family-friendly language, the topics are appropriate, and the survey questions are scientifically sound. Where appropriate, scientifically validated surveys that already exist and are well-accepted by the scientific community may be used in the registry.
-
How will my information be used by ACD?
ACD collaborates with researchers to share de-identified data to advance CCDS research and support development of possible treatments. Examples of this research may include assessing different genetic variants and their related symptoms, monitoring how the disorders progress over time, and supporting clinical treatment development. ACD will never share any personally identifying information entered into the registry. This includes first names, last names, and contact information.
-
I don’t have a confirmed CCDS diagnosis. Can I still enroll in the registry?
Currently, only patients and caregivers of individuals diagnosed with a CCDS are eligible to participate in this registry. If you are working on a diagnosis and would like support, please email registry@creatineinfo.org.
CreatineInfo at a Glance
Participate in the CreatineInfo Patient Registry!
Patients and Caregivers
- Join the registry
- Complete surveys
- Upload genetic and laboratory reports
Clinicians and Researchers
- Invite patients and families to participate
- Partner with ACD on a registry research project to learn more about creatine deficiencies
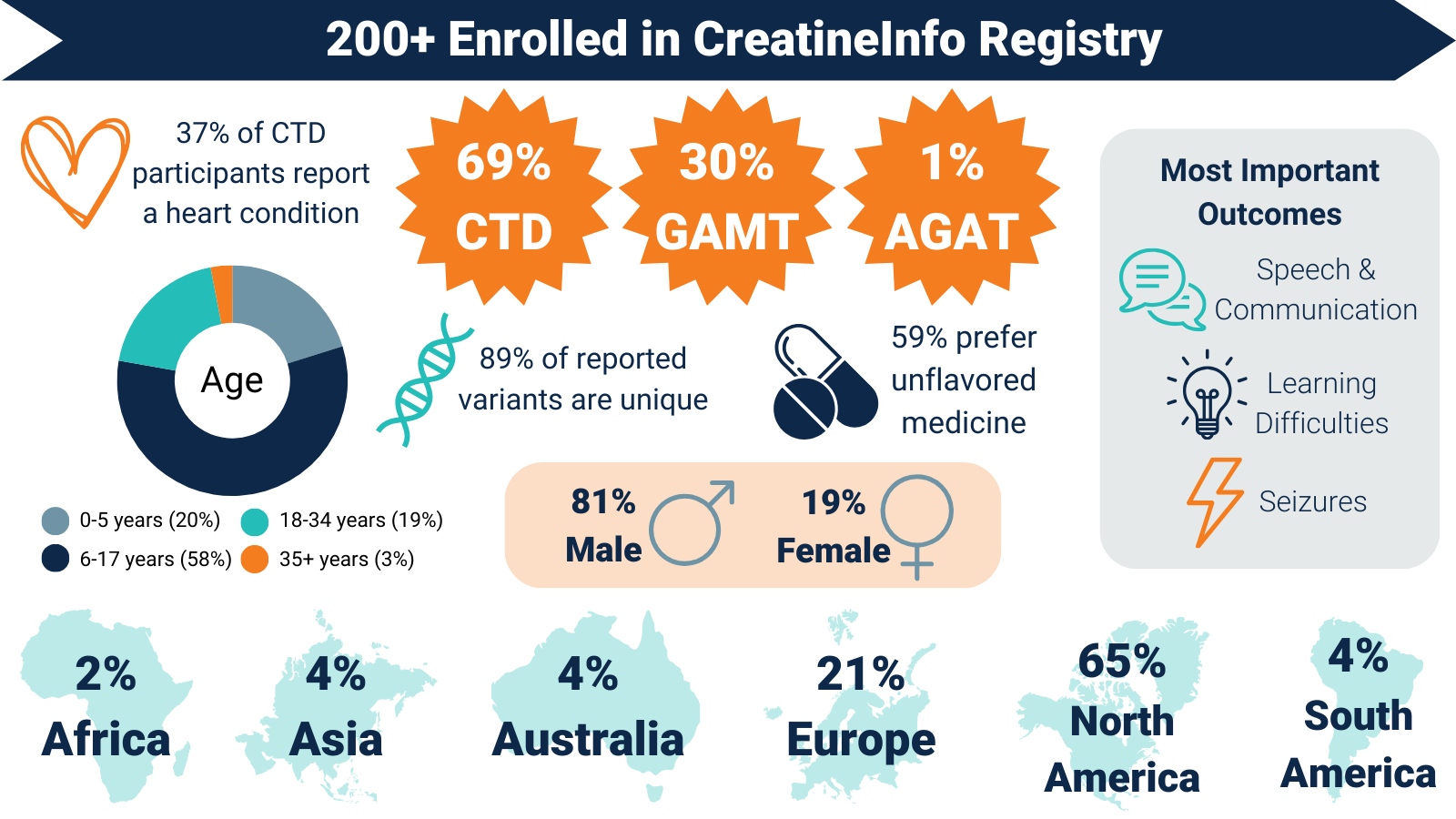
CreatineInfo Registry Results
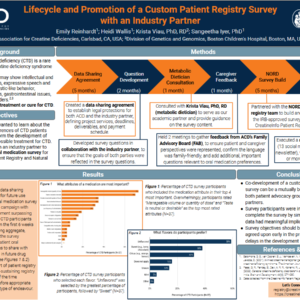
Lifecycle and Promotion of a Custom Patient Registry Survey with an Industry Partner
Poster presented at the NORD Rare Diseases and Orphan Products Breakthrough Summit, October 15-17, 2023, Washington, D.C., USA
ACD collaborated with an industry partner to develop a custom CreatineInfo survey to evaluate the oral medication preferences of CTD patients. In this poster, we discuss the goals of this project, our methods to develop a custom survey, and our preliminary findings.
CreatineInfo Patient Registry: Data in Action
Talk presented at ACD’s 2023 CCDS Virtual Conference, August 25-26, 2023
In this talk, ACD provides an update of the data currently available in the registry & natural history study, including results from the recently released oral medication preferences survey. This presentation also highlights the real-world uses of registry information which are utilized to advance CCDS research.
Talk presented at ACD’s 2022 CCDS Scientific & Patient Symposium, June 26-27, 2022, Park City, Utah, USA
In this presentation, ACD discusses the results of the patient meaningful outcomes survey. The goal of this survey was to learn from CCDS patients and caregivers the outcomes that are important to them and that they would like to see improved with a treatment for CCDS. Ultimately, these results can help improve clinical trial designs to better capture the patient and caregiver experiences.
The Creatine Info Registry: Demographics and Initial Findings
Talk presented at ACD’s 2022 CCDS Scientific & Patient Symposium, June 26-27, 2022, Park City, Utah, USA
ACD collaborated with Laura Voss, a master’s student in Genetic Counseling at the University of Utah. For her graduate research project, Laura examined the CreatineInfo Patient Registry, focusing on its development, launch, and initial findings.
Registry Regulations and Partnerships
-
Registry Advisory Board (RAB)
Responsibilities:
Discuss direction and goals of the registry and natural history study
Recommend possible surveys
Current Members:
ACD Board of Directors
Heidi Wallis, ACD Executive Director & Principal Investigator
Sangeetha Iyer, ACD Scientific Advisor
Emily Reinhardt, ACD Registry Coordinator -
Registry Oversight Board (ROB)
Responsibilities:
Set up registry, approve changes (IRB, protocol, consents)
Ensure limited direct data access (currently PI, registry coordinator, and ClinGen)
Review registry report proposals and what de-identified data will be included in any reports created
Current Members:
ACD Board of Directors
Heidi Wallis, ACD Executive Director & Principal Investigator
Sangeetha Iyer, ACD Scientific Advisor
Emily Reinhardt, ACD Registry Coordinator -
Family Advisory Boards (FAB)
Responsibilities:
FAB-Development (FAB-D): Review content and family-friendly language of surveys and materials
FAB-Localization (FAB-L): Review translations and accessibility of surveys and materials
Current Members:
FAB-D:
Unlisted
FAB-L:
Recruiting -
Institutional Review Board (IRB)
North Star Review Board serves as the independent IRB for the CreatineInfo Registry, providing ethical oversight and ensuring federal and institutional compliance of all human research conducted within the registry.
-
Data Security
The registry is hosted on the NORD IAMRARE® platform, which was developed with extensive input from experts at the National Institutes of Health (NIH), patient advocacy groups, and the U.S. Food and Drug Administration (FDA). The CreatineInfo Registry and Natural History Study is a secure platform, compliant with:
- U.S. health information privacy laws (HIPPA, HITECH, and FISMA)
- State privacy laws (where applicable)
- FDA regulations on electronic records (21CFR Part 11)
- European Union Data Protection Directive and General Data Protection Regulation (GDPR)
-
Protection of Participant Identity
To de-identify data, all protected health information (PHI) is removed. ACD follows the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule to define PHI. As we are a rare community, we also follow the cell size suppression policy as defined by the U.S. Department of Health and Human Services.
-
Access to Data
Direct data access is limited to the registry coordinator, the registry principal investigator, and ClinGen (when permission is granted). Requests for registry data must be approved by the Registry Oversight Board. Data sharing guidelines are outlined in the registry protocol, which has been reviewed and approved by the IRB. Any data reported are anonymous (de-identified) and aggregated (combined and summarized) with data from other registry participants.
-
ClinGen Data Sharing Program
ACD works with the Clinical Genome Resource (ClinGen) Patient Data Sharing Program to give registry participants the choice to share their de-identified and pseudonymized genetic and health data. ClinGen is a National Institutes of Health (NIH)-funded project aiming to define the impact of genes and genetic changes on health. You can view the consent form here.
-
Industry Partnerships
Custom registry surveys and access to de-identified data may be possible through a partnership with ACD. Please contact us for more information.
-
Industry Recruitment
The registry may facilitate collaboration between clinicians and industry partners, and assist in recruitment of participants for clinical trials. If industry partners wish to contact patients and families for clinical trials, ACD may contact those families on their behalf. ACD does not release personally identifiable information, including contact information, on any of its participants and their families.
-
Registry Protocol and Consent Forms
Protocol
Consent forms (adult, legally authorized representative)
Have questions? Contact Emily!
I am Emily, the Patient Registry Coordinator for ACD. If you have any questions, please email me at registry@creatineinfo.org or fill out the form below. I will follow up with you promptly.