Creatine Decoded: ACD-Funded CTD Drug Repurposing Fellowships Make Progress in Year One
Patient Samples from Coriell Biobank in Studies Seeking to Understand CTD Mutations & Explore Existing FDA-Approved Drugs as Potential Treatments
#CreatineDecoded is a quarterly educational essay series that sheds light on research relevant for Cerebral Creatine Deficiency Syndromes (CCDS). The essays and interviews feature community contributors, often parents, who with the help of the ACD, explore in their own words the CCDS science you want to know more about.
Have a topic in mind? Send suggestions to Laura Trutoiu, ACD Director of Research auract@creatineinfo.org.
Did you know that there may already be drugs out there that can help creatine deficiencies? In early 2021, the Association for Creatine Deficiencies (ACD) awarded a total of $90,000 in fellowship awards for three early career researchers studying Cerebral Creatine Deficiency Syndromes (CCDS). This Creatine Decoded article will highlight two of those projects, which are working collaboratively toward a common long-term goal of finding precision medicine treatments for Creatine Transporter Deficiency (CTD) via drug repurposing. An award for $30,000 funded Dr. Peter Axerio-Cilies, in the lab of Dr. Sylvia Stockler at BC Children’s Hospital Research Institute in Vancouver, and $30,000 of funding was awarded to Dr. Charles Kuntz, in the lab of Dr. Jonathan Schlebach at the University of Indiana, Bloomington.
The two fellowship projects complement each other in characterizing specific mutations and how they respond both in computer simulation and petri dish experiments to existing compounds.
Through these studies, research is identifying potential treatments targeting specific genetic variants by identifying repurposable drugs that can restore functional activity (creatine uptake) of the dysfunctional SLC6A8 transporters.
In our search for a cure and treatments for Cerebral Creatine Deficiency Syndromes (CCDS), ACD is playing an active role in initiating and participating in research including gene therapy, drug development, and drug repurposing. In 2021, ACD has taken a lead role in funding and pushing forward drug repurposing studies like this one. An ambitious Holiday Heroes fundraising goal of $250,000 by 2022 has been set to support these types of projects moving forward.
What is drug repurposing?
In layman’s terms, drug repurposing refers to finding an already existing medication that could help a condition other than the one it was initially designed for.
With drug repurposing, existing pharmaceutical drugs or supplements are identified and screened for effective use in the disorder being studied. According to the FDA (in a recent presentation by Heather Stone, “Capturing Clinician’s Experiences Repurposing Drugs to Inform Future Studies in the Era of COVID-19”), there are advantages to repurposing existing drugs. For instance, “time and cost to develop a new indication for an existing drug may be significantly less compared to developing a new drug from scratch because: most of the non-clinical drug development has already been done including chemistry, manufacturing and control, animal toxicology and clinical pharmacology.” Put simply, because these drugs have already been in humans for another disease indication, there is limited need to conduct safety studies and identify the best route of delivery, so these can be brought to market faster.
There is little financial incentive for companies to invest in the process of identifying repurposable drugs that may be available as generics, as it would not lead to large returns on their investment, but there is great potential for these drugs to help more than just the first disorder they were initially developed to treat. ACD sees this as an opportunity and is now funding multiple studies involving drug repurposing.
What research are the ACD fellows doing in drug repurposing?
Dr. Axerio-Cilies and Dr. Kuntz together with Dr. Stockler and Dr. Schlebach are leading the way in identifying drugs that might rescue creatine uptake across different types of SLC6A8 mutations. Their approaches are complementary and rely on combining two types of techniques: in silico or virtual screening and functional or cellular testing.
In silico screening is a computational method that utilizes the structure of a protein and screens the structure against a virtual library of millions of drug-like chemical structures. This process enables researchers to quickly understand if certain drug structures can bind to the protein efficiently. Once a drug binds to a protein, it can stabilize it and/or increase its function, both of which will be useful in rescuing the defect associated with CTD—reduced creatine in the cells.
In the first year, Dr. Kuntz’s work has focused on understanding the structure of SLC6A8 by modeling it computationally. Dr. Axerio-Cilies’ work focused on screening against libraries of drugs—both completely novel drugs as well as repurposable drug collections, to see if any of these drugs bound to the structure of SLC6A8.
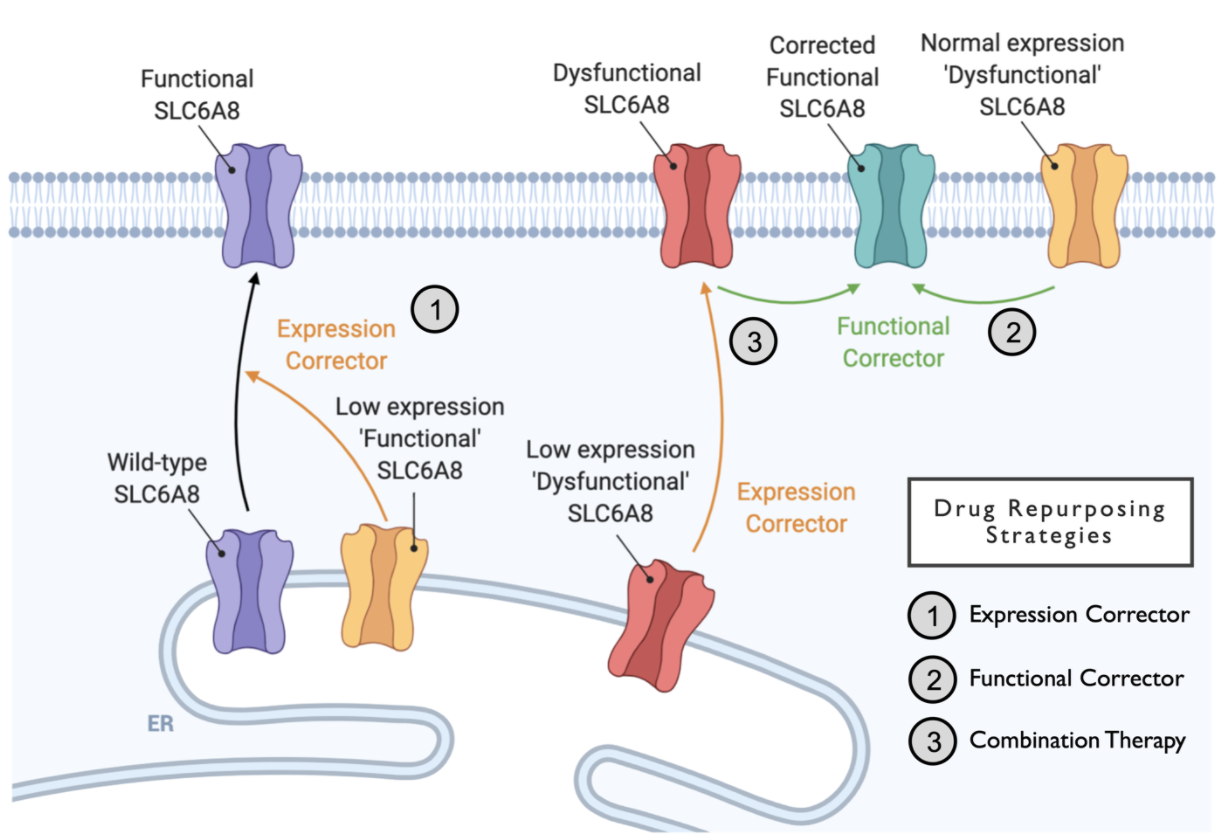
Next, both labs have generated cells that bear patient mutations. Drugs that bound to the structure of SLC6A8 computationally were then tested on these mutant SLC6A8-bearing cells. This type of testing is called functional or cellular function testing. Both Dr. Kuntz and Dr. Axerio-Cilies use complementary approaches to test the functional impact of drugs on different SLC6A8 mutations. Where Dr. Kuntz’s work tests if drugs can increase the expression of SLC6A8 protein, Dr. Axerio-Cilies’ work tests if drugs can increase the function of the SLC6A8 transporter by allowing more creatine in the cells. Below is a schematic that describes how different types of therapeutic approaches may be used to rescue the CTD defect.
The schematic summarizes the different ways mutations can affect SLC6A8 function and how they may be corrected. Some mutations can affect only the expression of the transporter protein—these are the low expression mutants. For this category, drugs that increase the expression of the transporter may be sufficient to restore function. Some mutations can affect function, but not expression. For these, drugs that stabilize or correct the function of the mutant transporter may be used to restore function. Finally, some types of mutations can affect both the expression and the function of the transporter. For these, using a combination of drugs that increase expression and also restore function may work best.
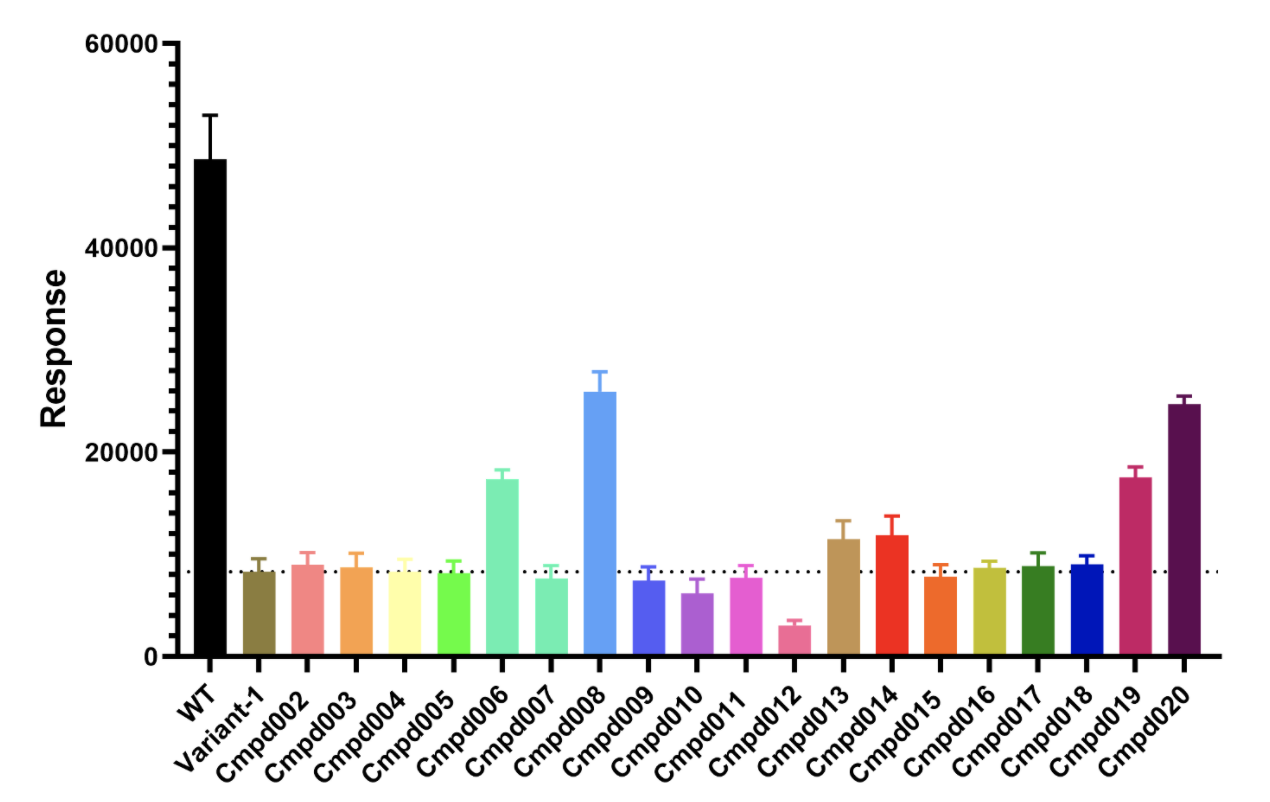
There are over 150 mutations that have been detected in patients with CTD. As you can imagine, finding the right type or types of drugs that can rescue the defect associated with this many mutations is no small feat. That is why a combination of approaches is necessary to arrive at a therapeutic option. By using in silico approaches, these scientists have been able to screen tens of thousands of potential drugs. They have also been able to test a handful of promising drugs in some patient variants. Below are examples of their results with two such patient variants.
This bar plot shows the creatine transport response of one patient variant or mutation in the presence of several drugs. For comparison, the very first black bar shows the creatine transport response of the wildtype (WT or healthy) SLC6A8 in the absence of any drugs. The second bar, labeled variant 1, shows the creatine transport due to a mutation-bearing dysfunctional SLC6A8. The remaining bars show the response of the same variant when it is treated with drugs. As you can see, compounds 6, 8, 13, 14, 19 and 20 are able to increase the creatine transport in this variant. Though these results are promising, they are just the beginning stages of finding a repurposable drug. There are several steps ahead until it may be possible to find a drug that can help our children. First and foremost, researchers need to consider safety and reproduce the results in actual patient cells. Additionally there are questions about what amount of medications are required and what level of increase we need to see to make a tangible and meaningful change.
In addition to finding that several non-functional mutations can be rescued by drugs, the researchers also found that four out of 15 tested mutations still retain the ability to transport small amounts of creatine. Such data can be instrumental in increasing our understanding of different patient mutations.
What’s coming up next?
The results above show that there are compounds out there that may increase creatine transport. Together, approaches by Dr. Axerio-Cilies, Dr. Stockler, Dr. Kuntz and Dr. Schlebach can help us find drugs that may rescue the deficit caused by mutations in SLC6A8. The next step would be to follow up on this finding by understanding the mechanism of rescue (expression or function), and collecting similar data on additional variants, followed by verifying this finding in patient fibroblasts bearing the same mutation.
Without patient fibroblasts, we have no way to confirm that this finding is relevant to physiological cells (i.e. cells that function as they would in your body). This is why it is extremely important for our community to deposit their patient fibroblast samples in the Coriell repository where they can be accessed by researchers like Dr. Axerio-Cilies and Dr. Kuntz.
We need the help of our entire community to continue funding audacious work like the current fellowships. ACD is currently raising funds through the Holiday Heroes campaign to continue supporting these fellowship projects, and more as part of a diverse pipeline of research efforts with the ultimate goal of clinical trials for CCDS patients. Make a donation today at creatineinfo.org/holidayheroes.
—
View recent roundtable discussion on drug repurposing: These researchers, supported by ACD fellowship awards, are making REAL progress towards a treatment for CTD. To help our community better understand the concept of drug repurposing for creatine deficiencies and the ongoing research in this area, we are pleased to share this new video featuring ACD fellows Dr. Schlebach and Dr. Axerio-Cilies about the concept of drug repurposing for creatine deficiencies, and how we can get to n=1 clinical trial and beyond with a repurposed drug for CTD.