Creatine Decoded: CCDS Gene Therapy Research
This essay was written by Laura Trutoiu, ACD Director of Research, with support from Erin Coller, ACD Director of Communications, and Sangeetha Iyer, ACD Scientific Advisor.
#CreatineDecoded is a quarterly educational essay series that sheds light on research relevant for Cerebral Creatine Deficiency Syndromes (CCDS). The essays feature community contributors, often parents, who, with the help of the ACD, explore in their own words the CCDS science you want to know more about. Have a topic in mind? Send suggestions to Laura Trutoiu, ACD Director of Research auract@creatineinfo.org.
When we got Rohan’s Creatine Transporter Deficiency (CTD) genetic diagnosis mid-2017 he was two and a half and had barely started to walk. Though sweet and loving, Rohan wasn’t using words and wasn’t quite doing what other two year olds do. It was a long road to a diagnosis and finding the genetic cause was a blow and a sigh of relief. I got the call at work and jumped out of an ordinary meeting into a brave new world full of genes and mutations.
I expected the genetic counselor to tell me they didn’t find anything, as they had with countless tests before. Instead, in an almost apologetic voice, I heard “We found a mutation in SLC6A8, the gene that produces the creatine transporter.” And there’s no treatment. And the outcome is not fatal but does lead to severe developmental and cognitive delays, often seizures, little to no speech, and a life forever dependent on a caregiver.
In the frantic days after the call I started reading all I could about genes and mutations. I’m an engineer and researcher with no medical background, but as a student of science I’ve seen the developments in gene therapy and all the promise it holds. There’s a long path though from scientific discovery to what we can actually do clinically. The good news is that with creatine deficiencies, we know where the error is and we know what it does: a single gene is affected, and there are a few people already studying it. Would gene therapy work for CTD and GAMT deficiency? In this essay, I’ll explore a bit of the basic science behind gene therapy. And full disclosure, I’m also writing this because, as a community, we need to continue to support gene therapy efforts for CCDS. You can be a Holiday Hero and help us fund the CCDS Gene Therapy Consortium for 2021.
In both CTD and GAMT deficiency, we have something to work with. Sure, it will take some time, some science and some $$$, but the initial conditions are in our favor. Of course biology is not as simple as hacking wires, but there’s a path and that’s what I’m discussing below from my perspective as a parent. For a great accessible read on genes, I highly recommend The Gene: An Intimate History, a bestseller and wonderfully written history of science by Siddhartha Mukherjee.
Throughout this essay, I use educational materials adapted from BioMarin Pharmaceuticals (BioMarin generously made these resources available for patient orgs like the ACD). Please keep in mind, the figures, materials and overall information below cannot be considered in any way as medical advice. Before we delve into gene therapy, here’s a bit of Biology 101.
Bio 101: Chromosomes, Genes and Mutations
Most cells in our body contain tiny structures called chromosomes, and most of us have 23 pairs of chromosomes which are made of DNA. DNA is the entire recipe book that determines our characteristics. A gene is a segment of DNA and we all have about 30,000. These genes are smaller individual recipes for the body to cook up proteins. Proteins are complex molecules that do all sorts of work in the body from helping blood clot to shuttling nutrients (like creatine) in and out of the cells. For example, the creatine transporter that the SLC6A8 gene produces is a protein that’s expressed on the cell surface (membrane-bound) to open up a channel for creatine to get into the cells. Many of us have a handful of mutations on some genes with no particular impact. Most of these mutations don’t matter, but in some cases they lead to dysfunction of the proteins they’re supposed to create. A mutation in a gene can lead to several things:
- Proteins do not work correctly
- Not enough proteins are made
- Too much protein is made
Note, it’s also possible that a mutation in the gene doesn’t do anything to the protein, in which case it is called a benign mutation. Mutations that have an effect are pathogenic. The type of mutation (which letters of the DNA are changed, missing or even added) determines which of these outcomes happen, but the mapping to outcomes is complex (doctors often talk about genotype to phenotype correlations, but that’s a topic for another essay). In CTD mutations, SLC6A8 is not made or doesn’t work properly. Some mutations are only one letter changed in the 3 billion pairs of DNA. Some are deletions and some insertions.
If you’re wondering how to read your own gene mutation, keep in mind that in 2021 the ACD is launching CreatineInfo, a new patient registry hosted on the NORD platform. As part of the registry you’ll have the opportunity to upload your genetic report. That will help track everyone’s mutations in the community and, longer term, what impacts there are according to which mutations we all have. Does it matter what mutation you have for gene therapy? Not really. The common version (see the next section) of gene therapy we talk about should theoretically work for all mutations, and as in all science, experiments are needed to fully confirm that.
Gene Therapy
When people talk about gene therapy there are several different types the words may refer to. CRISPR-CAS9 is a popular method of repairing or fixing a mutated gene. Cell therapy (like CAR-T therapy which is common in cancer) introduces a working gene into cells and then those cells are delivered into the body. But by far the most common reference, and the one we really care about in all three CCDS, is Gene Transfer, usually vector-based delivery, which introduces COPIES of the working, or functional gene into the body.
Some gene therapies are already approved for treatment of certain conditions, including a genetic eye disease and spinal muscular atrophy, but many more are currently being studied. If you’re like me, at this point you’re wondering where we are with investigational gene therapy research. Figure 1 shows a timeline of gene therapy. Since 2017 we are seeing gene transfer therapy approved for conditions in the US and a lot more in development.
Gene Transfer and Vector-Based Gene Therapy
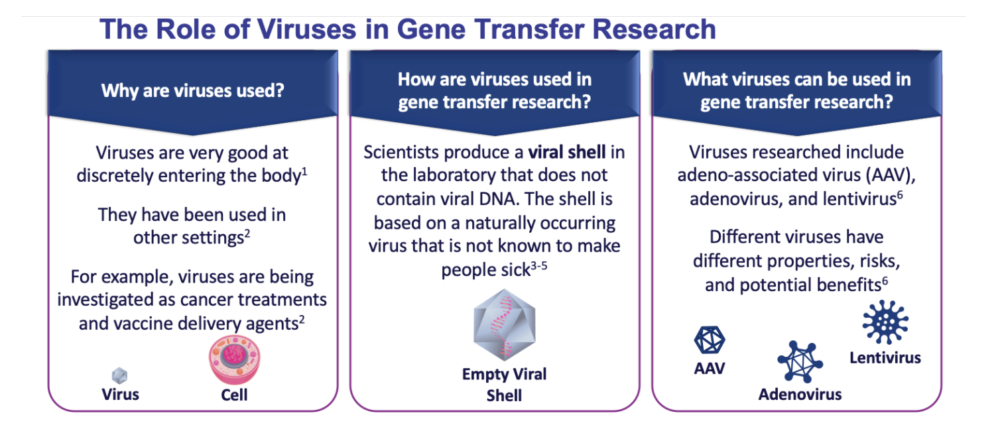
You can’t insert a gene directly into a cell because that gene would likely not work. Instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The viruses are modified so they can’t cause disease when used in people. The vector can be injected or given intravenously (by IV) directly into a specific tissue in the body, where it is taken up by individual cells. Figure 2 provides some details about why and how viruses are used as a vector delivery method in gene transfer therapies. For CTD, we need the vector to infect brain cells with a healthy SLC6A8 gene so that it starts producing the creatine transporter on the surface of the cell where it needs to transport creatine into the cells.
There are serious technical challenges before gene therapy will be a practical approach to treating any disease. For example, scientists must find good ways to deliver genes and target them to particular cells. For CTD, this means that the vector needs to reach the brain, and for GAMT, it needs to get into the liver. Another challenge is that whatever gene you deliver, you must also ensure that new genes are precisely controlled by the body and do not over-produce the protein or enzyme they’re responsible for.
Now for some good news: monogenic conditions, like GAMT deficiency and CTD, are good candidates for gene transfer research because only one functional gene is typically required. Even more importantly, the size of the SLC6A8 and GAMT genes is small enough that it is feasible to pack into one of the already known vectors. Can gene therapy help our kids if the clinical methods are years away? The simple answer is we don’t know but it gives us a shot and I’ll take those odds any day. Read on for where we’re headed and why.
How do we Pursue Gene Therapy Research for CCDS?
I started talking to various researchers right after Rohan’s diagnosis and I’d invariably ask about gene therapy. The frequent and honest answer is that gene therapy research is expensive and often takes multi-million dollar support over many years. As an example, the Rett Syndrome Foundation has invested over 10 million dollars in gene therapy research in 2019 only and this is the kind of funding that gets you to clinical trials. The Rett Syndrome Foundation work was the example that Dr. Steven Gray gave us when we asked what we can do to bring gene therapy to our community. Dr. Gray is a world renowned leader in gene therapy, especially for neurological conditions, and it was through initial conversations with him that we started the CCDS Gene Therapy Consortium that now has seven outstanding researchers (including Dr. Gray) interested in and pursuing gene therapy for CCDS.
The ACD’s fundraiser for gene therapy at the end of 2019 successfully raised $50,000 which allowed us to start the CCDS Gene Therapy Consortium. The goal of the consortium is to bring experts to the table to share insights and progress. Seeing such a strong and accomplished group of scientists, doctors and researchers come together to explore the potential for gene therapy for CCDS, as well as the strength of our community coming together to successfully fund initial efforts, brought a new level of hope and excitement to our family and community that I think was very much needed.
So what do researchers need to do to develop a gene therapy for CTD or GAMT deficiency? In general, one starts with designing a vector and a way to pack the gene you want into it. As we mentioned above, the vectors are viruses but they have to be engineered to carry the right payload and do no damage. Often, animal studies or cell studies are conducted to see how the gene therapy works before trying it in humans. Relatively speaking, designing the vector is one of the easy steps in the process. A lot more time and funding is required to produce and optimize the vector and ensure efficacy of treatment and safety.
The Gene Therapy Advancement Awards the ACD granted this past year went to two researchers, Dr. Laura Baroncelli and Dr. Gerrald Lipshutz, who both work with gene therapy for CTD and GAMT respectively in mice. The grant for Dr. Baroncelli is aimed at helping her lab learn from Dr. Steven Gray’s lab how to administer the vector in the spinal cord so that it eventually reaches the brain. Dr. Lipshutz is working on a GAMT gene therapy in mice. All the researchers in the consortium have active programs or are collaborating on gene therapy projects for CCDS. Our goal here is to make sure that we support our researchers with building block technologies, a bit like providing tiny legos (each grant is $10,000, so a small amount in the world of gene therapy) so that eventually we can build a full-blown castle.
The mission of the consortium is to facilitate the timely sharing of information and development tools among labs that are pursuing gene therapies for creatine deficiencies. We believe that by building a collaborative environment and supporting shareable tools through grants we can shorten the timeline and effort required to find gene therapy solutions for creatine deficiencies. The consortium meets on a quarterly basis as a group to discuss the latest research and provide peer expertise. Initial research is currently underway on gene therapy for CCDS, with ongoing projects by CCDS Gene Therapy Consortium members testing vectors such as AAV1 and AAV9 on mouse models.
During the 2020 ACD Virtual Conference, Dr. Jagdeep Walia, who is part of the CCDS Gene Therapy Consortium, presented a highly educational talk on how to develop gene therapy for CCDS. An example of what you might learn by watching the talk is that the best type of vector for treating CCDS is adeno-associated virus vector (AAV9). Also included in the talk is an estimated timeline of different types of studies that need to be completed. This timeline puts gene therapy development at over 6 years (Dosage study – 2 years, Route of administration study – 2 years, Immunosuppression – 1.5-2 years).
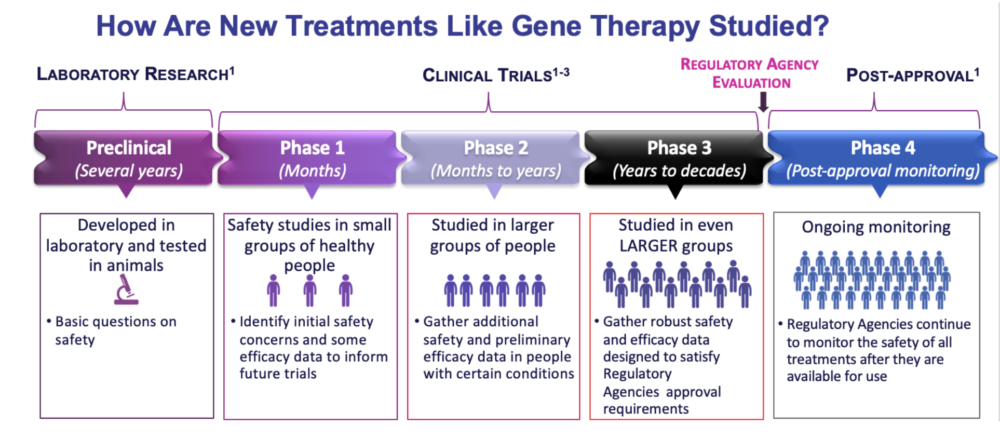
The big goal for gene therapy research for CCDS is to get to the clinical trial phase. Dr. Walia’s overview of this phase included the following stages: toxicology study, vector production, preparation for the application and approval and running the trial. Figure 3 also highlights how one generally gets to clinical trials, which should be familiar for all of us parents.
Aside from the need for large amounts of funding, one of the main challenges in developing gene therapy for CCDS is developing a strong working knowledge of how the creatine transporter functions in the body, how creatine is expressed in the brain and to other parts of the body and determining how to control the delivery of creatine into different types of cells without causing dangerous imbalances. The good news is that we learned through several presentations at the ACD 2020 Virtual Conference that many of these answers are forthcoming thanks to ongoing research.
We don’t know how much any of the therapy options out there, gene therapy or others, can help our kiddos, especially as they grow older. And we don’t know which therapies are going to succeed. We do know that restoring creatine in the brain makes a difference and patients show improvements. In AGAT deficiency, which is very similar to CTD and is treatable, replenishing the creatine in the brain resulted in patients of different ages making progress, though the later the treatment, the less cognitive improvement was observed.
So where are we now? We’ve started gene therapy research efforts and researchers are actively working on vectors and experiments in mice to see how much of the mice brain cells can be targeted by the vector and how much creatine increases. 2020 was a difficult year for all labs with delays in experiments, but overall a lot has happened and hopefully we can continue to fund the CCDS gene therapy consortium and see some of these results come to fruition. Rohan is still a happy kid and like all the adorable kiddos in our community, their chance at a better life is determined by whether we manage to push the scientific process, gene therapy and beyond, forward one lego brick at a time.
To help move the gene therapy research process along faster, here’s what you can do:
- Help us fund the CCDS Gene Therapy Consortium for 2021 and donate to our Holiday Heroes campaign. The goal is to keep awarding small grants to support early development for gene therapies.
- Donate biosamples to Coriell so researchers can work with each mutation.
- Upload your genetic reports into the upcoming CreatineInfo patient registry to ensure that our mutations are represented in larger datasets like ClinVar.
All thoughts and ideas expressed in the Creatine Community Blog represent the individual blog contributor’s opinions and not those of the Association for Creatine Deficiencies. The ideas expressed in the Creatine Community Blog, and any other locations on the creatineinfo.org website, should never be construed as medical advice, even if the information relates to the contributor’s actual health care experiences. Individuals should always follow the instructions of their physician and make no changes to their care unless instructed to do so by their physician.