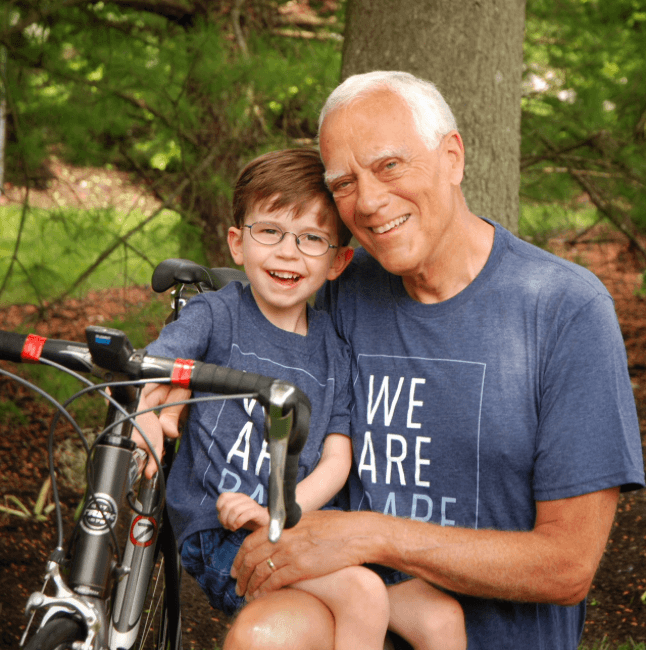“Drug Development- A Parent Perspective” – Erin
When the news came out about Lumos stopping the development of the drug they were working on to treat Creatine Transporter Deficiency (CTD), as a parent, it was a very emotional day and made me realize how important the drug development process is to me, to our family, and to our community. I recalled seeing a presentation at the inaugural ACD CCDS Scientific & Patient Symposium in 2018 regarding the drug development process that was helpful and eye-opening for me as a relative newbie to the world of pharmaceutical drug development.
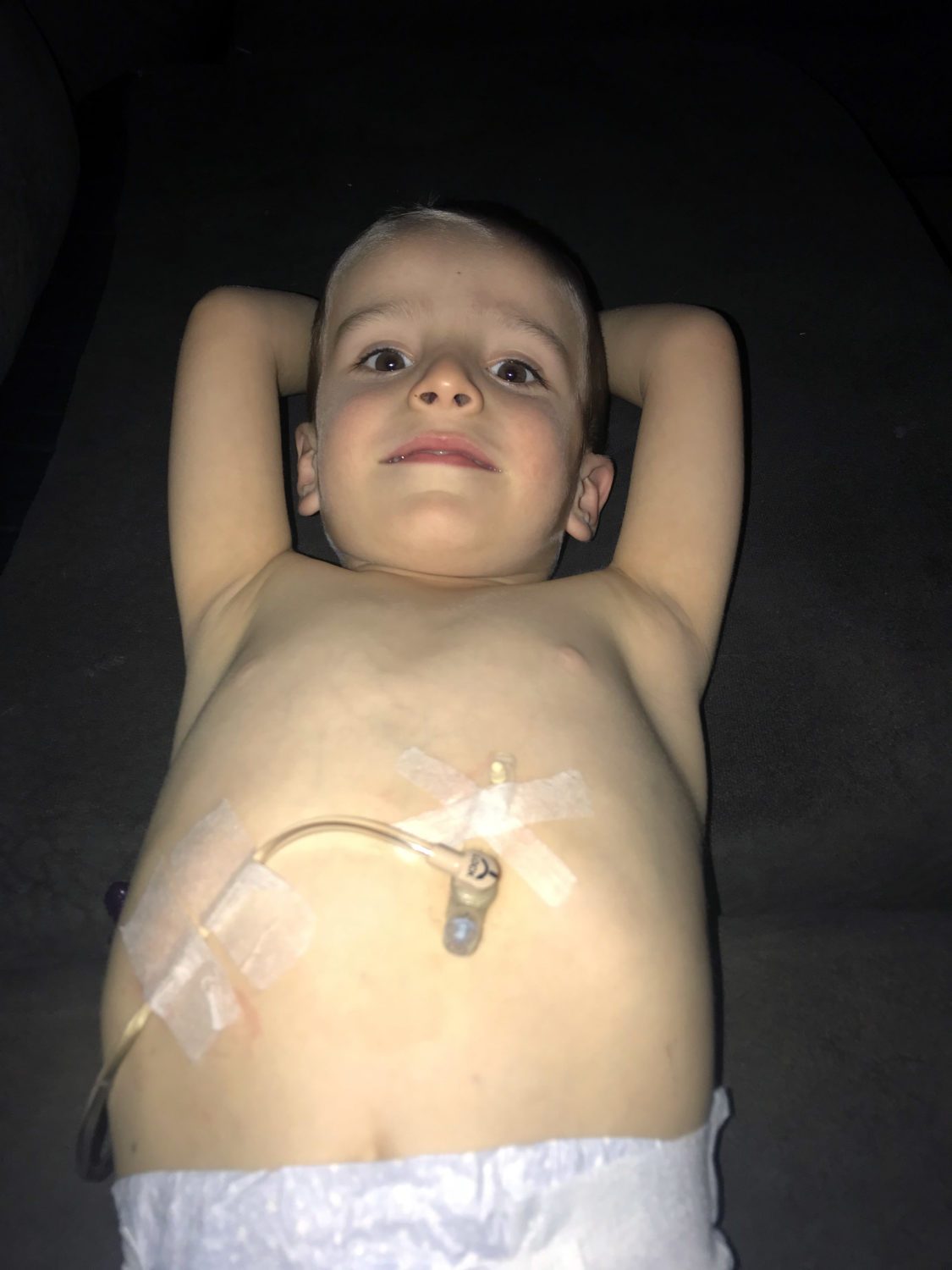
I was so happy and relieved to find out that the Vigilan study would continue to move forward and that Ultragenyx would take over the role as sponsor of this study. Most recently, Ultragenyx shared an update with our CCDS community regarding their role in the Vigilan study and their CTD clinical development program UX068. This was yet another reminder about all that I have yet to learn about this process.
For my husband and I, participating in the Vigilan study and being involved with the Association for Creatine Deficiencies as volunteers is extremely important to us, and we are so grateful to have opportunities to advocate for our son (Cadman, who is 4 years old and was diagnosed with CTD just before his second birthday), and play a role in the eventual development of a successful treatment for CTD. We have participated in the Ultragenyx online survey on CTD, and it was pretty quick and easy, and great to know we were helping contribute to the knowledge base of the team at Ultragenyx who are working hard to develop a CTD treatment that will hopefully make a meaningful difference in the lives of patients.
It can be overwhelming to try to understand the timeline and terminology involved in clinical drug development, as well as making sense of what all of it means for our son. In this presentation, Dr. Dave Weiner from Lumos does an excellent job of explaining the process. It is definitely worth the time to watch this if you have any questions about the drug development topic.
**Thanks to Erin Coller, ACD Ambassador, for writing this blog post.**